COMPANY
- Our company is located in Lithuania, the member of EU. We are working in a eld of party articles since 1996. Our produced moustaches and beards are ideal for parties, carnival, various shows, TV and cinema.
- Our company is located in Lithuania, the member of EU. We are working in a eld of party articles since 1996. Our produced moustaches and beards are ideal for parties, carnival, various shows, TV and cinema.
- All our live-size moustaches and beards are handmade, hand ventilated and combed.
- They are attached by means of a speci c medical plaster (on the back side of a moustache or a beard). This anti- allergic plaster does not irritate the skin, is easily stuck and removed.
- If necessary this moustache and/or the beard may be cut, combed or shaped by wet hands and xed by hair spray.
- For retail customers we offer card packing option.
- On bigger quantities customer’s package is welcome. We will gladly make custom moustaches and beards on all orders over 100 pcs.
OUR STORY
- We’re a family-run business selling handmade false moustaches and beards to wholesalers, stores and people all over the world. We’re based in the European Union, in Lithuania.
- Founders Tomas Siuipys and his wife Rasuole have been in the party industry for more 20 years. They’ve built a nationwide chain of party supply shops. At one point, unable to find really good fake facial hair, they began making it themselves – in all sorts of styles and colors.
- That was 6 years ago. Customers liked the newproducts so much that we decided to start offering them to the world. Now our fans are spread out across four continents and many countries, including the U.S.A. We have distributors in Europe, Canada, Australia and Japan, and also sell our ‘staches globally on Amazon.
- We’re a modern environmentally friendly company. Quality is our criteria No. 1. Creativity ensures everyone has fun. And great service makes life easier for our customers.
- The sticky fabric plaster, which contacts to the skin has CE certificate. It is classified according to Annex IX rules of the Medical Device Directive 93/42EEC as Class I device and is in accordance with Annex VII and all other applicable provisions of the Directive 93/43/EEC on the approximation of the laws of the European Union Member States concerning medical devices.
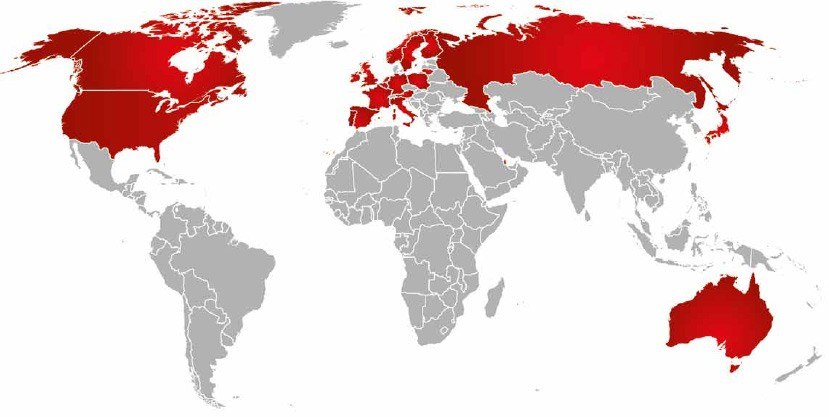
Our markets are in USA, Canada, Japan, Australia, Norway, Sweden, Finland, Lithuania, Poland, Russia, Germany, Belgium, Netherlands, France, Italy, Austria, Switzerland, United Kingdom, Ireland, Spain, Portugal, Qatar.

